-
Pfizer has submitted data on its bivalent COVID-19 booster shot that specifically targets the latest omicron subvariants. If authorized, the company says the shots could be ready as soon as September.
-
Despite the nation reaching the grim milestone of 1 million COVID deaths, ODH Director Dr. Bruce Vanderhoff says Ohio is doing "well" compared to previous spikes.
-
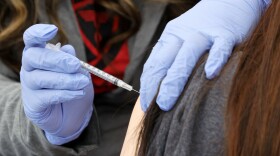
The Food and Drug Administration expanded authorization of Pfizer-BioNTech's COVID vaccine to enable kids ages 5 to 11 who were vaccinated at least five months ago to get a third shot.
-
The company warned consumers of several tainted lots of Accuretic and two other versions of the drug because of the presence of a nitrosamine above the acceptable daily intake level.
-
While approval would only be for those populations, an infectious disease specialist from Mount Carmel Medical Group says may be good for everyone.
-
Pfizer and BioNTech are planning to ask the Food and Drug Administration to authorize a second COVID-19 booster shot for people age 65 and older.
-
New research out of New York found the protection of the vaccine against infection in kids ages 5 to 11 dropped from 68% to 12%.
-
The new recommendation for adolescents age 12-17 came hours after a panel of CDC advisers voted in favor of it. The boosters should be given five months after initial immunization.
-
The move to shorten the Pfizer booster interval comes as the U.S. shatters daily case records. The recommended interval for those who received Moderna or Johnson & Johnson vaccines has not changed.
-
The authorization comes in the midst of an explosion of COVID-19 cases nationwide driven by the omicron variant — a surge that has brought a spike in pediatric hospitalizations.
© 2025 WOSU Public Media
Play Live Radio
Next Up:
0:00
0:00
Available On Air Stations